Note
Go to the end to download the full example code.
Vapor Dome#
This example generates a saturated steam table and plots the vapor dome. The steam table corresponds to data typically found in thermodynamic text books and uses the same customary units.
Requires: Cantera >= 2.5.0, matplotlib >= 2.0, pandas >= 1.1.0
temperatures correspond to Engineering Thermodynamics, Moran et al. (9th ed), Table A-2; additional data points are added close to the critical point; w.min_temp is equal to the triple point temperature
degc = np.hstack([np.array([w.min_temp - 273.15, 4, 5, 6, 8]),
np.arange(10, 37), np.array([38]),
np.arange(40, 100, 5), np.arange(100, 300, 10),
np.arange(300, 380, 20), np.arange(370, 374),
np.array([w.critical_temperature - 273.15])])
df = pd.DataFrame(0, index=np.arange(len(degc)), columns=columns)
df.T = degc
arr = ct.SolutionArray(w, len(degc))
# saturated vapor data
arr.TQ = degc + 273.15, 1
df.P = arr.P_sat / 1.e5
df.vg = arr.v
df.ug = arr.int_energy_mass / 1.e3
df.hg = arr.enthalpy_mass / 1.e3
df.sg = arr.entropy_mass / 1.e3
# saturated liquid data
arr.TQ = degc + 273.15, 0
df.vf = arr.v
df.uf = arr.int_energy_mass / 1.e3
df.hf = arr.enthalpy_mass / 1.e3
df.sf = arr.entropy_mass / 1.e3
# delta values
df.vfg = df.vg - df.vf
df.ufg = df.ug - df.uf
df.hfg = df.hg - df.hf
df.sfg = df.sg - df.sf
# reference state (triple point; liquid state)
w.TQ = w.min_temp, 0
uf0 = w.int_energy_mass / 1.e3
hf0 = w.enthalpy_mass / 1.e3
sf0 = w.entropy_mass / 1.e3
pv0 = w.P * w.v / 1.e3
# change reference state
df.ug -= uf0
df.uf -= uf0
df.hg -= hf0 - pv0
df.hf -= hf0 - pv0
df.sg -= sf0
df.sf -= sf0
print and write saturated steam table to csv file
T P vf ... sf sfg sg
0 0.010 0.006102 0.001000 ... 0.000000 9.157104e+00 9.157104
1 4.000 0.008116 0.001000 ... 0.060949 8.991303e+00 9.052252
2 5.000 0.008704 0.001000 ... 0.076093 8.950481e+00 9.026575
3 6.000 0.009331 0.001000 ... 0.091183 8.909950e+00 9.001133
4 8.000 0.010704 0.001000 ... 0.121196 8.829749e+00 8.950944
.. ... ... ... ... ... ... ...
69 370.000 210.174778 0.002217 ... 4.112145 6.921097e-01 4.804255
70 371.000 212.710748 0.002285 ... 4.143554 6.191883e-01 4.762742
71 372.000 215.280450 0.002376 ... 4.182301 5.296283e-01 4.711929
72 373.000 217.886235 0.002520 ... 4.236673 4.059971e-01 4.642670
73 374.136 220.890000 0.003256 ... 4.456452 4.751945e-07 4.456453
[74 rows x 14 columns]
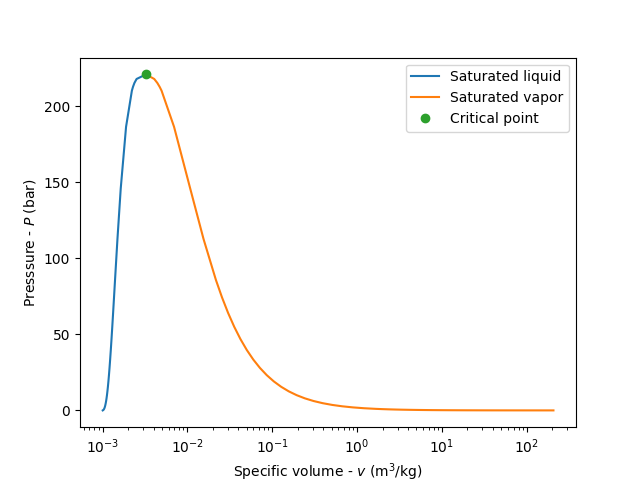
illustrate the vapor dome in a P-v diagram
plt.semilogx(df.vf.values, df.P.values, label='Saturated liquid')
plt.semilogx(df.vg.values, df.P.values, label='Saturated vapor')
plt.semilogx(df.vg.values[-1], df.P.values[-1], 'o', label='Critical point')
plt.xlabel(r'Specific volume - $v$ ($\mathrm{m^3/kg}$)')
plt.ylabel(r'Pressure - $P$ (bar)')
plt.legend();
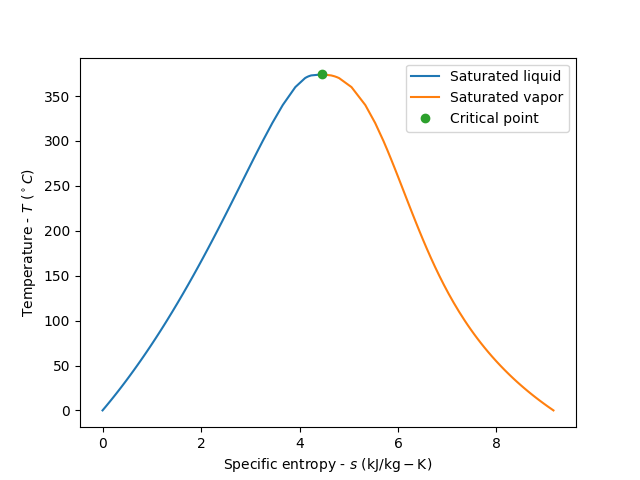
illustrate the vapor dome in a T-s diagram
plt.figure()
plt.plot(df.sf.values, df['T'].values, label='Saturated liquid')
plt.plot(df.sg.values, df['T'].values, label='Saturated vapor')
plt.plot(df.sg.values[-1], df['T'].values[-1], 'o', label='Critical point')
plt.xlabel(r'Specific entropy - $s$ ($\mathrm{kJ/kg-K}$)')
plt.ylabel(r'Temperature - $T$ (${}^\circ C$)')
plt.legend()
plt.show()
Total running time of the script: (0 minutes 0.616 seconds)

