Calculating Adiabatic Flame Temperature#
This guide demonstrates calculation of the adiabatic flame temperature for a methane/air mixture, comparing calculations which assume either complete or incomplete combustion.
Complete Combustion#
The stoichiometric equation for complete combustion of a lean methane/air mixture (\(\phi < 1\)) is:
For a rich mixture (\(\phi > 1\)), this becomes:
To find the flame temperature resulting from these reactions using Cantera, we create a
Solution object containing only the species in the above stoichiometric equations, and
then use the Solution.equilibrate function to find the resulting mixture composition
and temperature, taking advantage of the fact that equilibrium will strongly favor
conversion of the fuel molecule.
import cantera as ct
import numpy as np
# Get all of the Species objects defined in the GRI 3.0 mechanism
species = {S.name: S for S in ct.Species.list_from_file("gri30.yaml")}
# Create an ideal gas phase object with species representing complete combustion
complete_species = [species[S] for S in ("CH4", "O2", "N2", "CO2", "H2O")]
gas1 = ct.Solution(thermo="ideal-gas", species=complete_species)
phi = np.linspace(0.5, 2.0, 100)
T_complete = np.zeros(phi.shape)
for i in range(len(phi)):
gas1.TP = 300, ct.one_atm
gas1.set_equivalence_ratio(phi[i], "CH4", "O2:1, N2:3.76")
gas1.equilibrate("HP")
T_complete[i] = gas1.T
Incomplete Combustion#
In the case of incomplete combustion, the resulting mixture composition is not known in advance, but must be found by calculating the equilibrium composition at constant enthalpy and temperature:
Now, we use a gas phase object containing all 53 species defined in the GRI 3.0 mechanism, and compute the equilibrium composition as a function of equivalence ratio.
# Create an IdealGas object including incomplete combustion species
gas2 = ct.Solution(thermo="ideal-gas", species=species.values())
T_incomplete = np.zeros(phi.shape)
for i in range(len(phi)):
gas2.TP = 300, ct.one_atm
gas2.set_equivalence_ratio(phi[i], "CH4", "O2:1, N2:3.76")
gas2.equilibrate("HP")
T_incomplete[i] = gas2.T
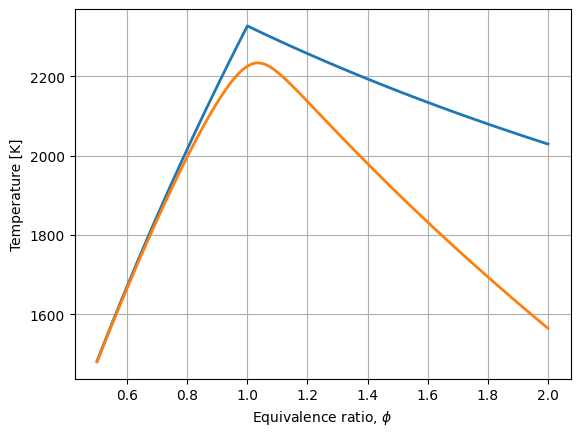
Comparing Results#
import matplotlib.pyplot as plt
plt.plot(phi, T_complete, label="complete combustion", lw=2)
plt.plot(phi, T_incomplete, label="incomplete combustion", lw=2)
plt.grid(True)
plt.xlabel(r"Equivalence ratio, $\phi$")
_ = plt.ylabel("Temperature [K]")